The Complexity of Natural Microbial Communities
Microbial Diversity
Microbial Diversity
Microbial Interactions
Microbial Interactions
Challenges in Laboratory Replication
Challenges in Laboratory Replication
Limitations of Growth Media
Nutrient Composition
Nutrient Composition
Static Conditions vs. Dynamic Nutrients
Static Conditions vs. Dynamic Nutrients
Environmental Control Challenges
Oxygen Gradients
Temperature and pH Dynamics
In conclusion, while laboratory control of environmental factors such as oxygen, temperature, and pH is essential for studying bacteria, these static parameters fall short of replicating the dynamic and heterogeneous conditions of natural ecosystems. The inability to mimic such variations limits the cultivation and understanding of many bacterial species, particularly those adapted to fluctuating environments. Recognizing these limitations is a critical step toward developing more sophisticated cultivation techniques that better capture the complexity of natural microbial habitats.
Emerging Technologies and Solutions
Co-Culture Systems
Co-Culture Systems
Microfluidic Technology
Microfluidic Technology
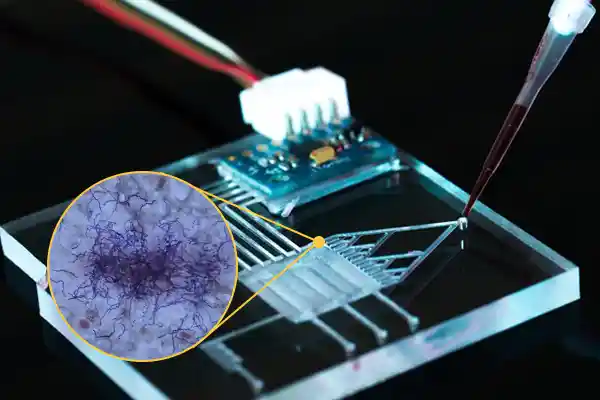
Metagenomics and Synthetic Communities
Metagenomics and Synthetic Communities
Conclusion
Growing perfect bacterial flora in the lab is challenging due to the immense complexity of natural microbial communities, limitations in traditional growth media, and the difficulty of replicating dynamic environmental conditions. However, emerging technologies like co-culture systems, microfluidics, and metagenomics offer promising solutions to overcome these barriers.
By improving our ability to cultivate diverse bacterial species and simulate natural ecosystems, researchers can unlock valuable insights into microbial ecology, human health, and environmental science. The pursuit of perfect bacterial flora may remain difficult, but ongoing innovations are bringing us closer to this goal.
What is Cell Therapy Manufacturing?Essentials and manufacturing processes for cell therapies
Cell therapy is rewriting the rules of modern medicine. Imagine a treatment that uses a patient’s own immune cells, reprogrammed in a lab to hunt down and destroy cancer—this is no longer science fiction [...]
Why use filter pipette tips? A Comprehensive Guide to Filter Pipette Tips
In laboratory workflows, unseen threats like aerosol contamination, residual liquid carryover, and cross-contamination can silently sabotage experimental results. This is where filter pipette tips step in—not just as disposable tools for liquid handling, but [...]
The Comprehensive Guide to Centrifuge Tubes
Centrifuge tubes are indispensable tools in modern laboratories, enabling the separation of samples by density through high-speed spinning. Widely used in scientific research, medical diagnostics, and industrial processes, these tubes play a critical role [...]