In today’s highly regulated laboratory environments, ensuring the accuracy, reliability, and compliance of instruments is not optional—it’s essential. This is where 3Q validation comes into play.
Comprised of Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), the 3Q process is the gold standard for validating laboratory equipment. Whether you’re working with analytical instruments like HPLCs or routine tools such as pipettes, 3Q validation provides a structured approach to ensure they perform as expected.
In this guide, we’ll break down each step of the 3Q process, explain its importance, and offer practical insights to help you implement it effectively.
What is IQ, OQ, PQ
In fact, regarding laboratory instrument validation, the most complete instrument and equipment validation solution actually includes 4 parts, namely 4Q: DQ (Design Qualification), IQ (Installation Qualification), OQ (Operational Qualification), and PQ (Performance Qualification).
However, since lab instruments are usually developed and designed by the manufacturers, the DQ phase is often not necessary in instrument validation.
1. Installation Qualification (IQ)
IQ is the first step in the 3Q validation process.
It focuses on verifying that the instrument has been delivered as specified, installed correctly, and set up according to the manufacturer’s requirements. This stage includes checking the equipment’s documentation, ensuring that all components and accessories are present, and confirming that the installation environment (e.g., temperature, humidity, electrical supply) meets the instrument’s specifications.
For example, during IQ for a high-performance liquid chromatograph (HPLC), technicians would inspect the system for completeness, verify that all tubing and detectors are properly connected, and confirm that the software and firmware are up to date.
2. Operational Qualification (OQ)
Once the equipment is installed, the next step is OQ.
This stage involves testing the instrument under a range of operating conditions to ensure it functions according to its design specifications. OQ typically includes a series of predefined tests to assess parameters like accuracy, precision, sensitivity, and reproducibility.
For example, in an HPLC system, OQ might involve checking the flow rate accuracy of the pump, ensuring the UV detector operates within its specified wavelength range, and confirming the consistency of sample injections.
This step is critical for identifying any operational issues that could affect the instrument’s performance.
3. Performance Qualification (PQ)
The final stage, PQ, evaluates whether the instrument performs consistently and reliably under actual working conditions.
Unlike OQ, which focuses on controlled tests, PQ emphasizes real-world scenarios that mimic the intended use of the instrument. For instance, an HPLC system undergoing PQ might analyze a series of test samples to verify that it delivers consistent results over time.
PQ ensures that the equipment not only meets theoretical specifications but also fulfills the practical needs of the laboratory.
In essence, 3Q validation serves as a comprehensive quality assurance mechanism for laboratory instruments.
By addressing the installation, operational performance, and real-world reliability of the equipment, it helps laboratories maintain high standards of accuracy, reproducibility, and compliance. Whether it’s a basic pH meter or a complex chromatographic system, 3Q validation provides confidence that the instrument is fit for purpose and capable of supporting critical scientific workflows.
Why IQ, OQ, PQ is Essential
The 3Q validation process—encompassing Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ)—is critical for ensuring laboratory instruments meet both regulatory requirements and operational standards.
Its importance lies in three key aspects: compliance, reliability, and efficiency.
1. Ensuring Compliance
In regulated industries such as pharmaceuticals, biotechnology, and food safety, compliance with standards like GxP, FDA, and ISO is non-negotiable.
3Q validation ensures that laboratory instruments are installed, operated, and maintained in a manner that meets these strict guidelines. This not only safeguards the integrity of experimental results but also ensures data traceability and audit readiness, helping laboratories avoid costly regulatory penalties or rejections.
2. Guaranteeing Instrument Accuracy and Stability
Laboratory instruments are only as reliable as their validation.
Through IQ, OQ, and PQ, the 3Q process confirms that instruments perform accurately and consistently across their lifecycle. This minimizes the risk of experimental errors caused by faulty equipment, ensuring the precision and stability required for generating high-quality data.
3. Reducing Risks and Improving Efficiency
Validated instruments reduce the likelihood of unexpected failures, downtime, or maintenance disruptions. By addressing potential performance issues early in the validation process, laboratories can lower operational risks and optimize workflow efficiency. This translates to improved productivity, higher data quality, and confidence in the reliability of results.
In summary, 3Q validation is not just a regulatory requirement—it’s a foundational practice for maintaining accuracy, reliability, and operational excellence in the laboratory. It enables researchers to focus on their work with the assurance that their instruments will deliver dependable results.
Which Instruments Need IQ, OQ, PQ
Not all laboratory instruments require 3Q Validation.
Some simple instruments, such as ultrasonic cleaners and electric furnaces, can be omitted because they are simple and have no direct impact on the test results.
For general instruments such as balances and rapid moisture meters, since they are not precision instruments, but the instrument status can have a direct impact on the test results, the 3Q Validation of such instruments will combine IQ and OQ into one step. In addition, PQ is confirmed after running for a period of time. Generally, manufacturers only provide PQ reference documents, which are freely performed by customers. Therefore, manufacturers only provide IQ and OQ most of the time.
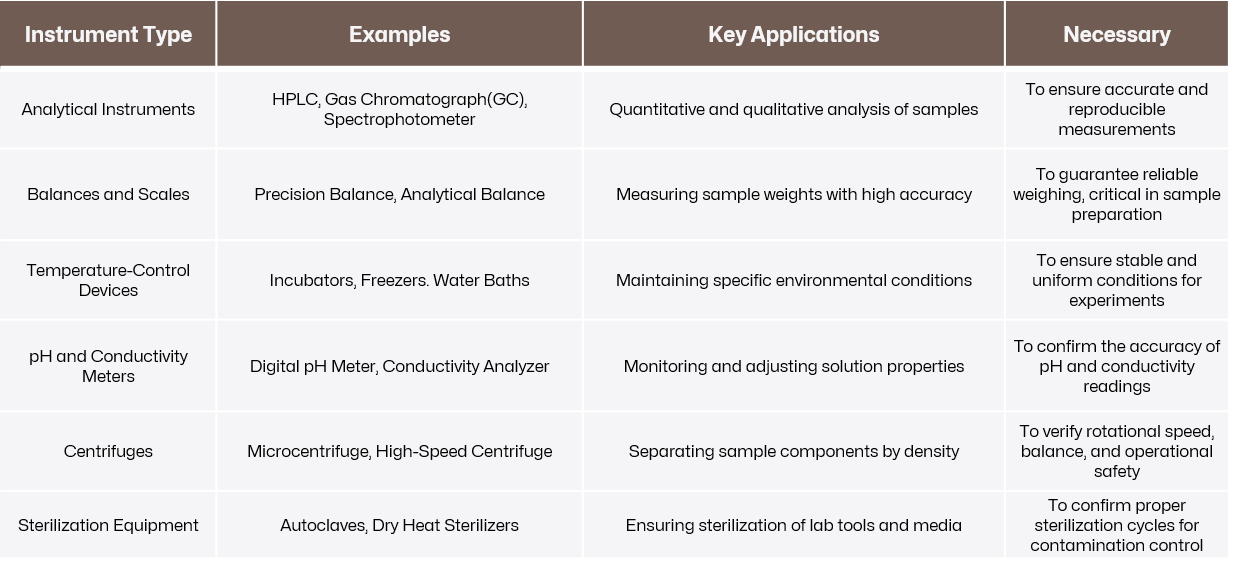
Precision instruments that are complex, critical to experimental results, or used in regulated environments often require 3Q validation. Below is a breakdown of common instrument types that often undergo IQ, OQ, and PQ, as well as their roles in the lab and why validation is critical for them.
Click to view original image
Factors Influencing the Need for 3Q Validation
1. Instrument Complexity
Instruments like high-performance liquid chromatographs (HPLCs) and spectrophotometers involve multiple components and intricate mechanisms, requiring careful validation to confirm proper installation and functionality.
2. Application and Usage Environment
Equipment used in sensitive applications, such as pharmaceutical testing or clinical diagnostics, where precision and reliability are critical, must undergo thorough validation. For example, incubators in microbiology labs must maintain exact temperature and humidity levels to ensure accurate experimental results.
3. Regulatory and Industry Standards
In regulated industries like pharmaceuticals, biotechnology, and food safety, standards such as those from the FDA, ISO, or USP mandate validation of critical equipment. These instruments play a vital role in ensuring product safety, efficacy, and compliance.
Any instrument that directly impacts experimental outcomes, regulatory compliance, or data integrity should be considered for 3Q validation. By validating these instruments, laboratories can maintain accuracy, avoid costly errors, and ensure compliance with industry standards.
How to Perform 3Q Validation
Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) provide a systematic approach to ensuring laboratory instruments perform reliably and consistently. Each phase builds upon the previous one, addressing specific aspects of the instrument’s setup, functionality, and real-world performance.
Below is a detailed breakdown of how to effectively carry out each stage of 3Q validation.
1. Installation Qualification (IQ)
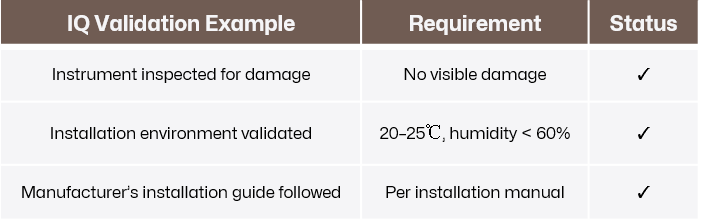
Verify that the instrument has been delivered, installed, and configured according to the manufacturer’s specifications and the laboratory’s requirements. Proper installation is the foundation of the validation process, as any errors at this stage can compromise subsequent steps.
Steps for IQ Execution
Inspection and Inventory:
Confirm that all components, accessories, and documentation are present and undamaged upon delivery.
Verify that key documents, such as calibration certificates, user manuals, and shipping lists, are included.
Environmental Checks:
Ensure the installation environment meets the specified requirements, such as temperature, humidity, and electrical supply. For instance, an HPLC system might require 20–25°C room temperature and a stable power source.
Installation and Setup:
Install the instrument following the manufacturer’s instructions, ensuring all connections (e.g., tubing, cables, and power supply) are correctly made.
Double-check mechanical alignments, such as detectors, pumps, and sample injectors.
Documentation:
Record details such as the instrument model, serial number, and installation date in an IQ report. Include photos of the setup, as well as diagrams of connections if applicable.
Click to view original image
2. Operational Qualification (OQ)
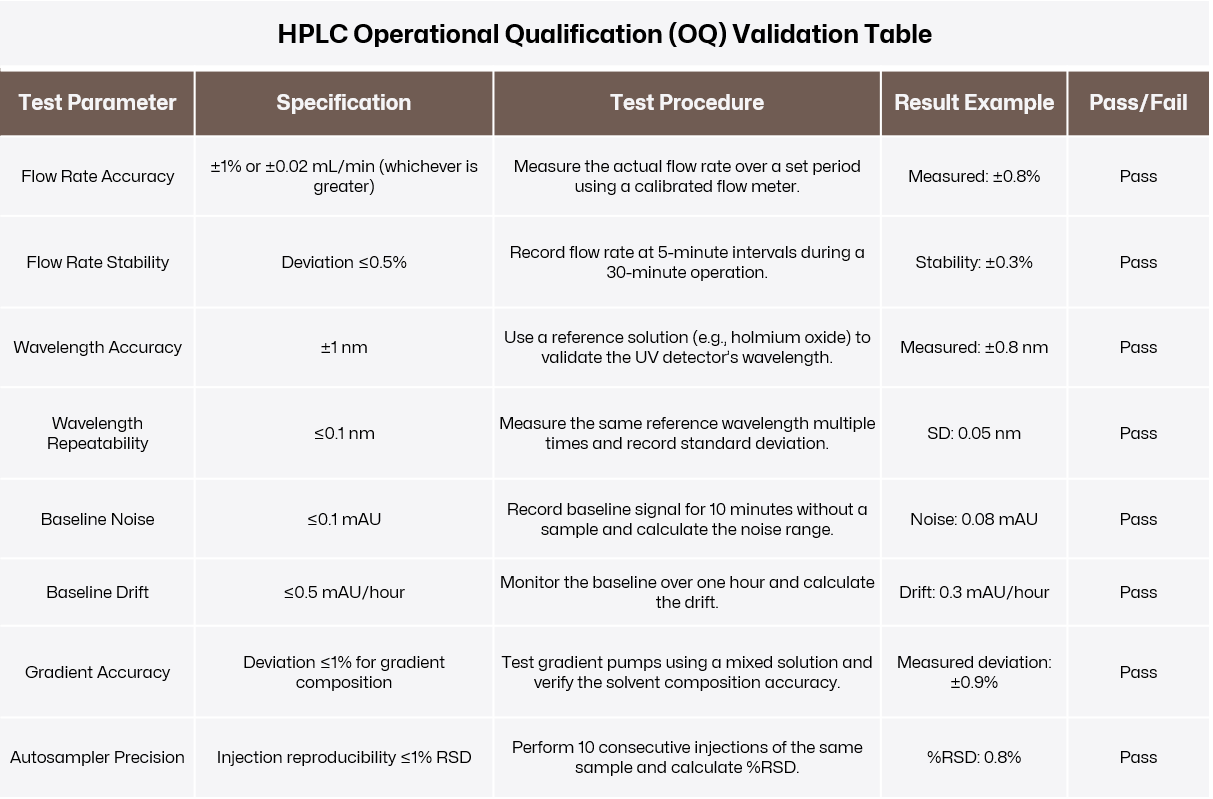
Confirm that the instrument functions as intended under controlled conditions. OQ tests the system’s performance against predefined parameters to ensure all critical components operate within acceptable limits.
Steps for OQ Execution
Initial Calibration:
Calibrate the instrument using certified standards. For example, an HPLC’s flow rate and detector wavelength can be calibrated with traceable reference materials.
Functional Testing:
Evaluate key performance metrics such as flow rate accuracy, wavelength accuracy, baseline noise, and stability. These tests ensure that the instrument’s core functions meet the manufacturer’s specifications. For an HPLC, test the pump’s flow rate accuracy to ensure deviations are within ±1%.
Safety and Alarm Verification:
Test the safety features, such as error alarms for temperature deviations or pressure limits, to confirm proper functionality.
Documentation:
Record results for each parameter in the OQ report. Note any deviations from the specifications and outline corrective actions taken, if necessary.
Click to view original image
3. Performance Qualification (PQ)
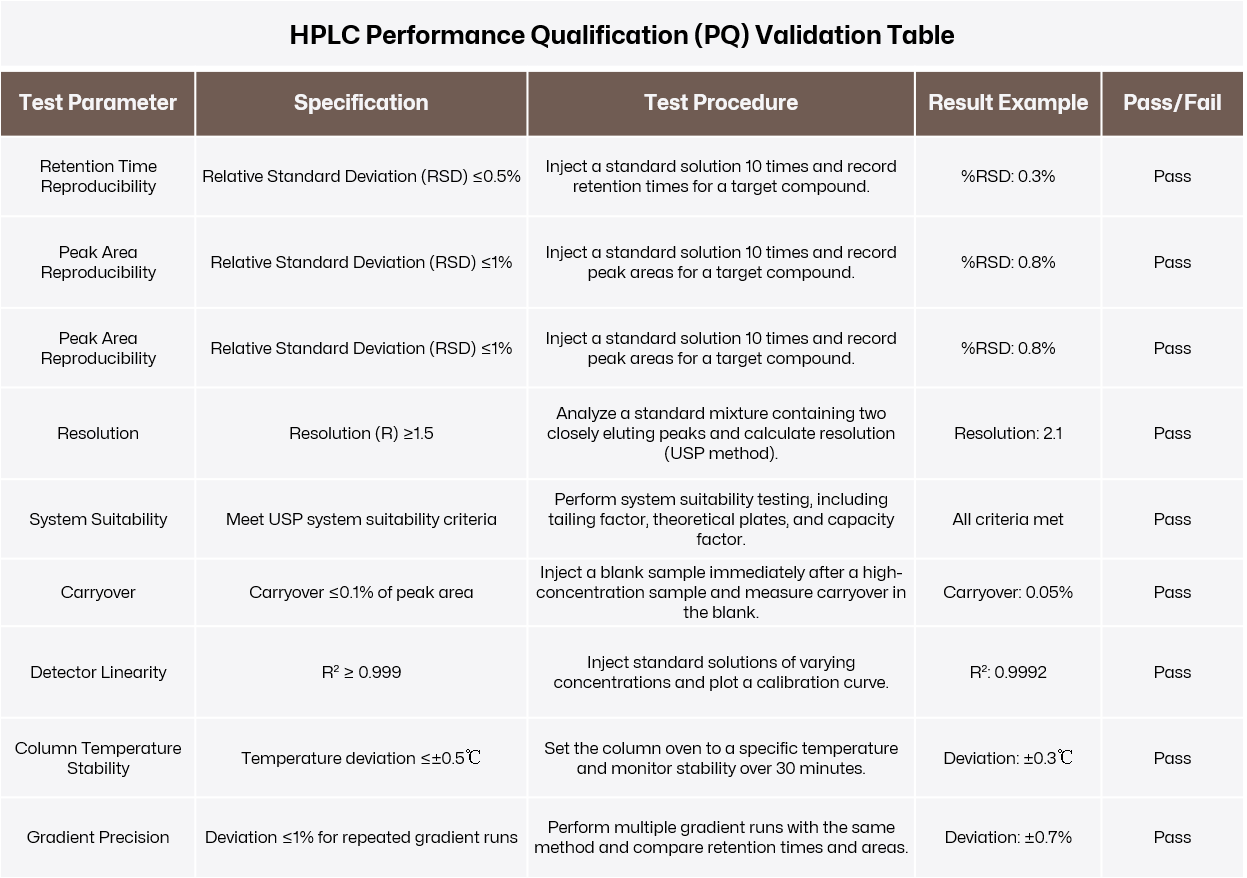
Verify that the instrument performs consistently and reliably under real-world conditions. PQ demonstrates that the equipment meets the laboratory’s operational needs during routine use.
Steps for PQ Execution
Real-World Testing:
Use the instrument to perform tasks representative of its intended applications. For example, inject standard solutions into an HPLC system to evaluate retention time reproducibility, peak area consistency, and resolution.
Stress Testing:
Run the instrument at its operational limits or over extended periods to evaluate long-term reliability. For instance, test a thermal cycler by running repeated cycles at its maximum temperature setting.
Data Analysis:
Compare the results against predefined acceptance criteria, such as %RSD (Relative Standard Deviation) for retention times or peak areas.
Documentation:
Record all results, deviations, and corrective actions in the PQ report. Ensure sufficient evidence is provided to support the instrument’s suitability for routine use.
Click to view original image
By systematically performing IQ, OQ, and PQ, laboratories can ensure that their instruments are properly installed, function as intended, and deliver reliable results under real-world conditions. Thorough documentation at each stage not only ensures compliance with regulatory requirements but also provides a comprehensive record of the instrument’s validation history. This robust process minimizes downtime, enhances data integrity, and instills confidence in the laboratory’s operations.
Common Issues and Solutions in 3Q Validation
3Q validation is a meticulous process, but challenges can arise during any stage, from Installation Qualification (IQ) to Performance Qualification (PQ). Common issues often stem from incomplete documentation, improper installation, or inconsistencies in validation standards. These challenges, if left unresolved, can lead to delays, non-compliance, or unreliable instrument performance.
1. Incomplete or Disorganized Validation Documentation
Validation requires comprehensive documentation, including IQ, OQ, and PQ protocols, test results, and deviation records. Incomplete or poorly managed documents can lead to gaps in traceability and compliance, especially during audits.
Solution:
Implement a Centralized Documentation System: Use document management software to store and organize all validation records in a single location. This ensures accessibility and prevents loss of critical information.
Standardize Protocol Templates: Develop and use standardized templates for IQ, OQ, and PQ to ensure consistency across different instruments.
Conduct Periodic Documentation Reviews: Assign dedicated personnel to review validation documents regularly for completeness and accuracy.
2. Improper Installation of Equipment
Instruments that are not installed according to manufacturer specifications or environmental requirements can fail validation tests during the OQ and PQ stages. Common errors include incorrect electrical connections, unstable setups, or insufficient environmental controls.
Solution:
Follow Manufacturer Guidelines: Ensure the installation is performed strictly according to the manufacturer’s instructions, including any specific environmental conditions such as temperature, humidity, or vibration limits.
Conduct Pre-Installation Assessments: Evaluate the laboratory environment beforehand to confirm it meets the instrument’s requirements.
Verify Installation with Checklists: Use detailed IQ checklists to confirm all components are correctly installed and connections are secure.
3. Inconsistent Validation Standards
Discrepancies in validation standards, such as varying acceptance criteria for performance parameters or conflicting regulatory requirements, can create confusion and lead to inconsistent results.
Solution:
Align with Industry Guidelines: Base validation protocols on recognized standards such as USP, ISO, or FDA regulations. For example, ensure HPLC validation follows USP system suitability criteria.
Establish Internal Validation Standards: Develop a uniform set of validation criteria tailored to your lab’s specific needs and ensure all personnel adhere to these standards.
Consult with Suppliers: Collaborate with instrument manufacturers to clarify any ambiguities in performance specifications or recommended validation procedures.
4. Time-Consuming Manual Validation Processes
Manual validation processes are labor-intensive and prone to human error, leading to inefficiencies and delays, particularly in laboratories with multiple instruments requiring validation.
Solution:
Adopt Automation Tools: Use validation management software to automate repetitive tasks such as data collection, parameter monitoring, and report generation. For example, software-integrated HPLC systems can automatically record and analyze test results.
Utilize Electronic Signatures: Implement systems that support electronic signatures to streamline approval workflows and reduce administrative overhead.
Schedule Validations Strategically: Develop a validation schedule that prioritizes critical instruments and allocates resources effectively to minimize downtime.
5. Challenges in Handling Deviations
Deviations from expected outcomes during validation tests are common, but failing to address them systematically can lead to unresolved performance issues and delays in completing validation.
Solution:
Develop a Deviation Management Plan: Create a formalized process for identifying, documenting, and resolving deviations.
Perform Root Cause Analysis (RCA): Investigate deviations thoroughly to determine their root causes and implement corrective actions.
Maintain Detailed Records: Document all deviations and their resolutions in the final validation report for traceability and audit purposes.
Use Validation Software: Invest in software designed for validation tracking, which can integrate with laboratory information management systems (LIMS). This simplifies record-keeping, progress tracking, and audit readiness.
Standardize Protocols Across Instruments: Uniform validation protocols reduce variability and improve consistency, even when validating different types of equipment.
Leverage Vendor Support: Collaborate with instrument manufacturers to obtain pre-written IQ/OQ templates and guidance on validation procedures.
Conduct Regular Internal Audits: Periodic audits can identify inefficiencies or gaps in the validation process, allowing corrective actions to be taken proactively.
Conclusion
IQ, OQ, and PQ are the cornerstones of laboratory instrument validation, providing a systematic framework to ensure compliance, reliability, and efficiency. These processes confirm that instruments are properly installed, function as intended, and deliver consistent results under real-world conditions. By adhering to 3Q validation protocols, laboratories can minimize errors, maintain regulatory compliance, and optimize workflows, ultimately safeguarding the integrity of their experimental data.
At GenFollower, we understand the importance of reliable laboratory operations. As a trusted supplier of high-quality laboratory plastic consumables and equipment, we are committed to supporting your lab’s success. Whether it’s through providing durable and precise products or offering solutions tailored to your specific needs, GenFollower is here to help you achieve accuracy and efficiency in every experiment.
to explore what reliable and efficient product we can offer you.What is Cell Therapy Manufacturing?Essentials and manufacturing processes for cell therapies
Cell therapy is rewriting the rules of modern medicine. Imagine a treatment that uses a patient’s own immune cells, reprogrammed in a lab to hunt down and destroy cancer—this is no longer science fiction [...]
Why use filter pipette tips? A Comprehensive Guide to Filter Pipette Tips
In laboratory workflows, unseen threats like aerosol contamination, residual liquid carryover, and cross-contamination can silently sabotage experimental results. This is where filter pipette tips step in—not just as disposable tools for liquid handling, but [...]
The Comprehensive Guide to Centrifuge Tubes
Centrifuge tubes are indispensable tools in modern laboratories, enabling the separation of samples by density through high-speed spinning. Widely used in scientific research, medical diagnostics, and industrial processes, these tubes play a critical role [...]