Have you ever faced inconsistent PCR results due to poor sealing, or wasted hours manually handling single tubes in high-throughput workflows? These challenges not only delay your experiments but can also compromise data accuracy. A 2022 study published in Nature Methods revealed that nearly 30% of qPCR errors originate from tube or plate incompatibility—underscoring the critical impact that consumable selection has on experimental success.
Choosing the right format—whether PCR tubes, strips, or plates—can be a game-changer. The correct format not only minimizes risks like cross-contamination but can also boost your throughput by as much as 50%. With a streamlined workflow, you can reduce manual errors and ensure more consistent, reliable results.
This guide can help you eliminate the confusion and provide you with a data-driven comparison of PCR tubes, strips, and plates. By understanding how each format influences efficiency and accuracy, you can optimize your experiments and overcome common pitfalls. Let’s explore the best practices to enhance your PCR workflow and achieve superior outcomes in your research.
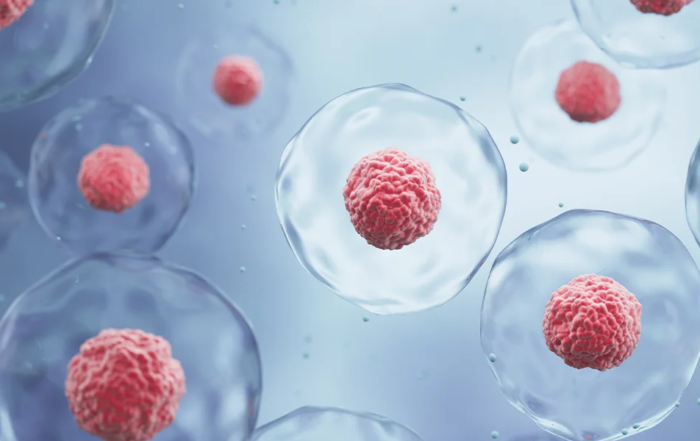
PCR Tubes vs. Strips vs. Plates: Performance, Pros, and Ideal Use Cases
PCR Single Tubes – Flexibility for Small-Scale Precision
Advantages:
Ultimate Flexibility: Ideal for individual reactions, pilot studies, or protocols requiring unique thermal profiles.
Minimal Cross-Contamination Risk: Single-use design avoids sample interaction.
Cost-Effective for Low Throughput: No need for specialized equipment (e.g., strip/plate adapters).
Limitations:
Low Throughput: Manual handling slows workflows (e.g., 30+ minutes to prepare 50 samples).
Higher Contamination Risk: Frequent cap opening increases aerosol exposure.
Thermal Inconsistency: Thin-walled variants may struggle with rapid cycling.
Best For:
Small-scale experiments (≤20 reactions).
Sensitive applications (e.g., long amplicons, nested PCR).
Labs with limited automation.
Your Content Goes Here
PCR Tube Strips – Middle Ground for Moderate Workflows
Advantages:
Balanced Throughput: Process 8–12 samples simultaneously (compatible with multi-channel pipettes).
Reduced Contamination: Fewer cap openings vs. single tubes (NIH reports 27% lower aerosol risk).
Automation-Ready: Compatible with most thermal cyclers and liquid handlers.
Limitations:
Fixed Format: Cannot process partial strips without wasting wells.
Sealing Variability: Poorly designed caps may leak during cycling (critical for low-volume reactions).
Best For:
Medium-scale workflows (50–200 samples/day).
Routine diagnostics or genotyping.
Labs transitioning to semi-automation.
PCR Plates – High-Volume Efficiency for Automated Systems
Advantages:
Maximized Throughput: Process 96–384 samples in one run (ideal for robotics).
Superior Thermal Uniformity: Industrial-grade molding ensures ≤0.5°C inter-well variation.
Low Evaporation: Heat-sealing films reduce volume loss by 90% vs. manual capping.
Limitations:
High Initial Cost: Requires plate-compatible cyclers, seals, and automation tools.
Overkill for Small Studies: Unused wells increase per-sample costs.
Best For:
Large-scale projects (e.g., NGS library prep, population studies).
Labs with full automation (e.g., robotic liquid handlers).
Time-sensitive workflows (e.g., pandemic surveillance).
How to Choose Between PCR Tubes, Strips, and Plates: A 3-Step Decision Guide
Step 1: Define Your Experimental Scale
Small-scale (1–50 reactions/day): Prioritize single tubes for flexibility and low upfront costs.
Medium-scale (50–300 reactions/day): Opt for strips to balance speed and manual handling.
High-throughput (300+ reactions/day): Invest in plates for automation compatibility and bulk pricing.
Example: A lab processing 150 COVID samples daily reduced setup time by 65% by switching from tubes to strips.
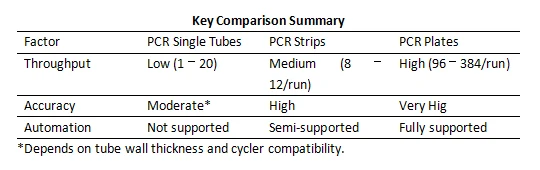
Step 2: Assess Automation Capabilities
Step 3: Evaluate Accuracy Requirements
Low-risk workflows (e.g., routine genotyping): Strips or tubes suffice.
High-sensitivity applications (e.g., qPCR, rare allele detection):
Use plates with uniform wall thickness (≤0.2 mm variation) to prevent edge effects.
Choose single tubes for protocols requiring individualized thermal optimization.
Data Insight: Plates reduce inter-well temperature variation by 80% compared to manual tube setups (Journal of Biomolecular Techniques, 2021).
Decision Flowchart
Start → Sample Volume (Low/Medium/High) → Automation (None/Partial/Full) →Accuracy Needs (Standard/High) → Recommended Format.
Example Workflow:
NGS library prep (1,000 samples, automated) → 384-well plates.
Student training (20 samples, manual) → single tubes.
Key Considerations for Optimizing PCR Experiments with Tubes, Strips, and Plates
1. Validate Seal Integrity to Prevent Evaporation and Contamination
Testing Methods:
Visual Inspection: Check for gaps between caps and tubes/strips under a microscope.
Dye Test: Fill wells with colored solution, invert the plate/strip, and monitor leakage after centrifugation (500 × g, 2 min).
Thermal Stress Test: Cycle consumables 5× between 4°C and 95°C, then measure volume loss (>5% indicates poor sealing).
Pro Tip: Use pre-certified consumables (e.g., our PCR plates with laser-etched sealing ridges) to bypass manual checks.
2. Ensure Thermal Cycler Compatibility
Critical Checks:
Thickness Match: Verify tube/strip/plate wall thickness aligns with your cycler’s specifications (e.g., 0.2 mm variance can reduce heat transfer by 15%).
Adapter Requirements: Semi-skirted plates may need specific frames; strips require compatible racks.
Edge Effect Mitigation: For plates, avoid using outer wells for critical reactions unless your cycler has uniform block heating.
Example: A mismatched 0.3 mm-thick tube caused a 22% drop in target yield in a 2023 BioTechniques study.
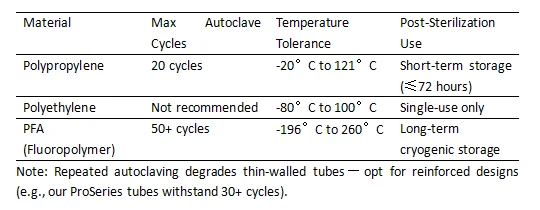
3. Follow Sterilization Limits to Avoid Warping
4. Minimize Contamination in High-Throughput Workflows
Prevention Tactics:
Pre-Aliquot Reagents: Use strips/plates pre-filled with master mix to reduce pipetting steps.
Seal Once, Open Never: For plates, apply heat-sealing films instead of reusable caps.
UV Treatment: Decontaminate racks and plate holders between runs (30 min UV exposure).
Red Flag: Aerosol contamination risk increases by 3.7× when reopening strips/tubes mid-run (NIH, 2022).
5. Optimize Storage Conditions Post-Experiment
Short-Term (<1 week): Store sealed strips/plates at 4°C with desiccant packs.
Long-Term (>1 month):
For tubes: Use screw-capped variants and store at -20°C.
For plates: Vacuum-seal with moisture barrier films to prevent evaporation.
FAQs about PCR consumables
Q1: Can I cut PCR strips into individual tubes?
While technically possible, cutting strips compromises seal integrity and increases risks:
Evaporation: Exposed edges lose up to 15% reaction volume during cycling.
Contamination: Rough edges trap aerosols (NIH reports 2.5× higher contamination risk).
Thermal Inefficiency: Uneven tube walls disrupt heat transfer.
Q2: Are all PCR plates compatible with my thermal cycler?
No. Compatibility depends on:
Skirt Type (full/semi/non-skirted): Check your cycler’s manual for plate specifications.
Well Volume: Standard 96-well plates (200 µL) differ from low-volume variants (50 µL).
Material Thickness: Polypropylene plates must match block contact specifications (±0.1 mm).
Q3: How do I prevent edge effects in PCR plates?
Edge wells often underperform due to temperature fluctuations. Mitigate this by:
Preheating the Block: Let the cycler equilibrate for 10 minutes before starting.
Avoiding Outer Wells: Reserve edge wells for controls or blanks.
Using Uniform-Wall Plates: Our PCR plates feature a uniform 0.30mm thin wall, enabling fast and uniform heat conduction.
Q4: Can I autoclave PCR tubes and plates repeatedly?
It depends on the material:
Polypropylene (PP): Withstands 20 autoclave cycles (121°C, 15 psi) but may warp after 10+ uses.
Polyethylene (PE): Not recommended for autoclaving—opt for single-use.
PFA Fluoropolymer: Autoclave-safe for 50+ cycles (ideal for reusable high-end tubes).
Warning: Warped tubes reduce sealing efficiency by 40%. Always inspect pre-sterilized consumables.
Q5: How do I reduce cross-contamination in high-throughput workflows?
Follow these WHO-recommended protocols:
Pre-Fill Master Mix: Use pre-aliquoted strips/plates to minimize pipetting.
Keep sealing: Apply adhesive films or heat seals immediately after loading—never reopen.
UV Decontaminate: Expose racks and lids to UV light for 20 minutes between runs.
Q6: Why does my reaction volume decrease in tubes/strips after cycling?
Evaporation is the primary culprit. Combat it by:
Using Tight-Fitting Caps: Test seals with the dye method.
Adding Overlay Oil: For long protocols (>2 hours), add 10 µL mineral oil.
Choosing Thin-Walled Formats: Our PCR single tubes, tube strips and plates ensure extremely low evaporation rates even at 95°C compared to standard designs.
Conclusion
Choosing between PCR tubes, strips, and plates isn’t just about convenience—it’s about precision, efficiency, and reproducibility. Whether you’re scaling up for high-throughput genomics or refining small-scale assays, the right consumables can:
Boost throughput by 50% with automation-friendly plates.
Achieve accurate results through ultra-thin-wall PCR tube strips with excellent thermal conductivity.
Cut long-term costs through optimized thermal performance and reduced repeat experiments.
Take Action Now:
Request Free Samples: Test our PCR single tubes, tube strips, or plates in your lab.
What is Cell Therapy Manufacturing?Essentials and manufacturing processes for cell therapies
Cell therapy is rewriting the rules of modern medicine. Imagine a treatment that uses a patient’s own immune cells, reprogrammed in a lab to hunt down and destroy cancer—this is no longer science fiction [...]
Why use filter pipette tips? A Comprehensive Guide to Filter Pipette Tips
In laboratory workflows, unseen threats like aerosol contamination, residual liquid carryover, and cross-contamination can silently sabotage experimental results. This is where filter pipette tips step in—not just as disposable tools for liquid handling, but [...]
The Comprehensive Guide to Centrifuge Tubes
Centrifuge tubes are indispensable tools in modern laboratories, enabling the separation of samples by density through high-speed spinning. Widely used in scientific research, medical diagnostics, and industrial processes, these tubes play a critical role [...]